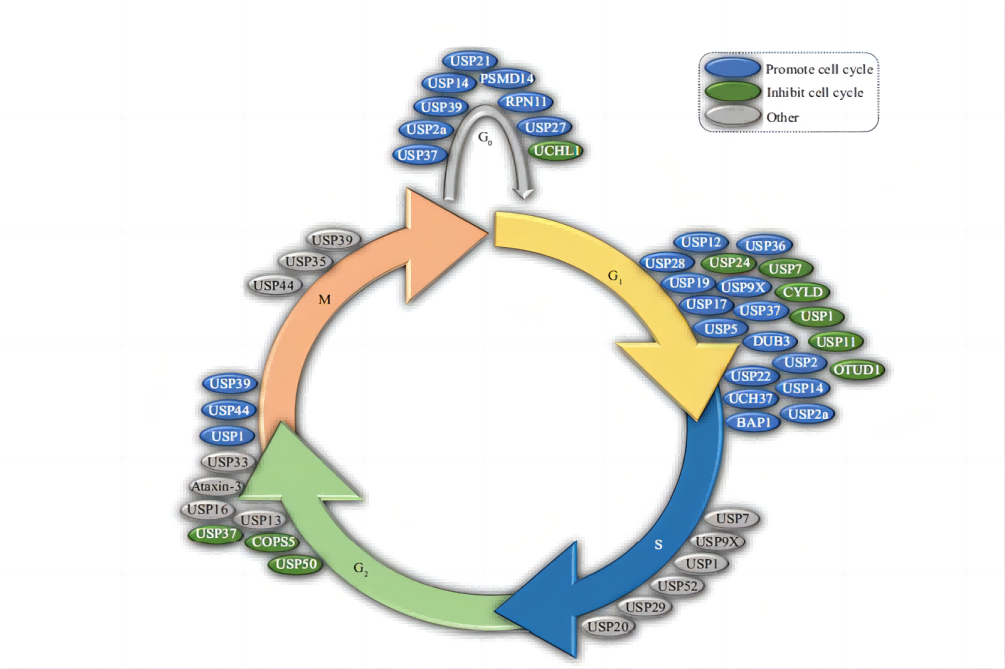
Zhang Zairong's research group at the Interdisciplinary Research Center of Biology and Chemistry at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences found that misfolded membrane proteins undergo a "re-ubiquitination" process after they are ubiquitinated and transported from the endoplasmic reticulum membrane to the cytoplasm. In turn, it can be effectively recognized and degraded by the proteasome. Relevant research results were recently published in the journal Molecular Cell.
The accumulation of misfolded proteins in cells can cause damage to cells, cause cellular dysfunction and lead to diseases, such as neurodegenerative diseases. In order to maintain normal physiological functions, eukaryotic cells have evolved the ubiquitin-proteasome pathway to degrade misfolded proteins: the protein substrate to be cleared is covalently modified by ubiquitin molecules under the action of ubiquitin ligase, and then protease recognized and degraded by the body. In addition, cells rely on the kinesin p97 complex to clear misfolded transmembrane proteins. The p97 complex separates it from the membrane, unfolds it, and transports it into the hydrophilic cytoplasm, where it is recognized and degraded by the proteasome located there. However, the process of how membrane proteins are recognized by the proteasome after being separated from the biological membrane by the p97 complex, unfolded, and released into the cytoplasm has been overlooked.
Zhang Zairong's team selected gout-related misfolded membrane protein ABCG2 as the research object and found that ABCG2 transported into the cytoplasm by the p97 complex will be recognized by a complex composed of the molecular chaperone BAG6 and the ubiquitin ligase RNF126. The study further found that RNF126 mediates the re-ubiquitination of the substrate protein, allowing the substrate protein to be effectively recognized and degraded by the proteasome. This strategy by which cells degrade misfolded membrane proteins is called "reubiquitination," which promotes rapid degradation of protein substrates transported or unfolded by p97 and reduces the retention of peptide chains unfolded by p97 in the cytoplasm. time so that the substrate does not form aggregates and cause damage to the cells. At the same time, this study revealed that the substrate range of RNF126-BAG6 is broad-spectrum, indicating that "reubiquitination" may be a ubiquitous mechanism. The "reubiquitinating enzyme" RNF126 is highly expressed in a variety of cancer cells, can promote cell growth, and is likely to be closely related to the function of cancer cells to clear misfolded proteins.